Background
Acute myeloid leukemia (AML) still remains a significant challenge, with intensive chemotherapy treatments showing limited efficacy and lack of druggable targets in contrast to other hematologic malignancies. Exosomes, nanomolecules derived from cells reflect the parent cell's state, present opportunities for disease biomarker discovery and targeted therapy. Proteomic analysis of AML-derived exosomes may identify druggable targets despite limited prior research. In this study we investigated the potential of exosomes as a tool for target discovery in AML.
Methods
We conducted a proteomic analysis of AML-derived exosomes from HL-60, KG-1, and THP-1 cell lines, using fibroblast and mesenchymal stem cells as controls. Exosomes of each cell line were isolated, and digested via filter-aided sample preparation (FASP), followed by isobaric tagging using tandem mass tag (TMT) reagents. The samples were then fractionated using high-pH reversed-phase liquid chromatography and analyzed using Q-Exactive Orbitrap Hybrid Mass Spectrometry. Proteomic data were evaluated using SEQUEST in Proteome Discoverer 2.1 with a false discovery rate set to 1%. Differentially expressed proteins (DEPs) were identified and visualized in an interaction network and was analyzed for the gene ontology biological process (GOBP) using DAVID software. Protein-protein interactions were analyzed using the STRING software.
Based on proteomic analysis, we identified the BTK-related pathway as potential druggable target for AML cells. AML cells were incubated with ACT, a BTK inhibitor, in various exposure time and density. Cell viability and proliferation rate was assessed using CCK-8 assay. BTK activity and signaling were assessed via Western blot analysis and immunocytochemistry of AML-derived exosomes. Cell death was evaluated using Annexin-V & PI apoptosis kit and FACS analysis. Statistical analysis was performed to ascertain significance.
Results
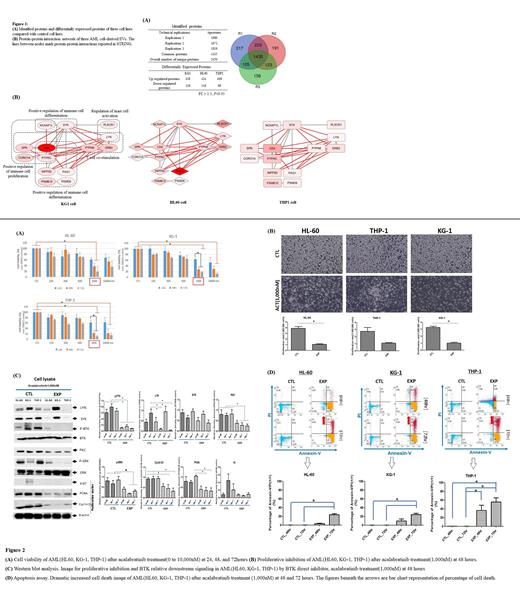
Proteomics of exosomes from three AML cell types revealed distinct protein profiles via LC-MS, with 2450 unique proteins identified (Figure 1A). For KG1, HL60, and THP1, the number of up-regulated DEPs were 438, 434, and 408, and the number of down-regulated DEPs was 126, 118, and 89 (fold change>1.5 or <0.67, P<0.05). Gene ontology analysis identified four main categories of biological processes, including cell migration, T-cell pathways, cell damage, and vesicle formation pathways.
Investigation into protein-protein interactions using the STRING software allowed selection of tyrosine kinases SYK and LYN as biomarker candidates. Both kinases regulate BTK, a critical molecule in B-cell antigen receptor signaling. SYK and LYN were found to be enriched in exosomes, promoting phosphorylation of BTK in recipient cells. (Figure 1B)
Cell viability experiments with AML cell lines revealed reduced viability upon treatment with ACT. Viability decreased with increased exposure time and density, with the highest effects observed at 1,000nM ACT concentration for 48 hours (Figure 2A). This was accompanied by decreased cell numbers and proliferation rate which was significant in the HL-60 and KG-1 cell lines (Figure 2B).
Western blot analysis demonstrated significant decrease of the BTK downstream signaling (P-BTK, PKC and p-ERK) by acalabrutinib treatment (1,000nM) at 48 hours (Figure 2C). Proliferative markers, such as PCNA, and Cyclin D1 were also significantly decreased. LYN and SYK was also decreased in the exosomes of HL-60 and THP-1 cell line. The inhibition of P-BTK and Cyclin D1 by ACT was further confirmed through Immunofluorescence assay. Finally, ACT was shown to promote cell death in AML cell lines, as evidenced by an apoptosis assay, with the most significant increases observed in cells treated exposed to 1,000nM ACT for 72 h (Figure 2D).
Conclusion
Overall, the findings of this study revealed that acalabrutinib reduced proliferation and induced cell death in AML cells suggesting the potential of BTK inhibitors in AML treatment. This was explained through proteomic analysis of AML-derived exosomes. However, further studies are required to validate the clinical use of exosomes as biomarkers and druggable target.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Acalabrutinib is used in this study to assess the proliferation and cell death of AML cell lines

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal